
The next step is to determine an antithrombotic regimen. This step is the most complex as there are benefits and risks, which vary based on patient profile. The first step should be to determine the patient’s bleeding risk. There are risk scores for bleeding in patients undergoing coronary stenting (precise dual antiplatelet therapy); however, there are few data in patients with PAD. General considerations include a known bleeding diathesis, need for systemic anticoagulation for another indication such as atrial fibrillation, prior hospitalization for bleeding, history of intracranial bleeding or stroke, and anemia as a marker of occult blood loss. These factors along with careful clinical assessment can help in consideration of global bleeding risk. In addition, few studies have evaluated the efficacy and safety of more potent antiplatelet therapies in patients with PAD and end-stage renal disease.
For all patients with PAD, low-dose aspirin or clopidogrel is recommended unless there is a contraindication. There are data to support efficacy of each of these agents, and clopidogrel was shown to be superior to aspirin in the CAPRIE trial and may have better gastrointestinal tolerability. The EUCLID trial demonstrated that the efficacy and safety of ticagrelor was similar to that of clopidogrel. For patients at high bleeding risk, additional antithrombotic agents are not recommended.
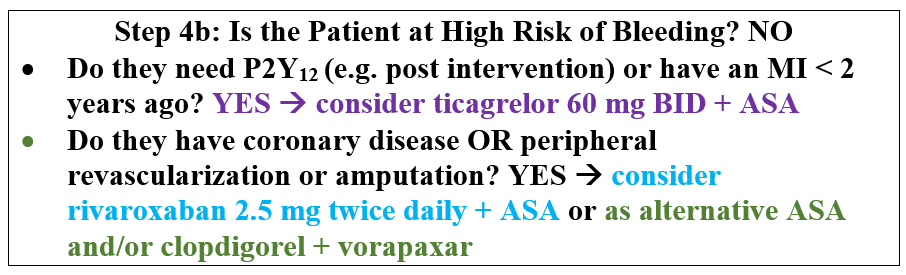
For patients who are not at high bleeding risk, determining whether or not to add additional agents depends on the risk profile. Currently there are three approved add-on therapies in patients with PAD. Although vorapaxar is FDA approved in patients with PAD, consensus guidelines indicate that the overall clinical benefit of adding this medication to existing antiplatelet therapy with symptomatic PAD is uncertain. Ticagrelor 60 mg twice daily is approved for patients with prior MI including those with concomitant PAD for reducing MACE and MALE. Rivaroxaban 2.5 mg twice daily added to aspirin was recently studied in the COMPASS trial and showed reductions in MACE and MALE as well as amputation.
All of these therapies increased major bleeding but did not increase fatal bleeding or intracranial hemorrhage. While in general there does not appear to be heterogeneity of benefit, clinicians may desire to identify patients at the highest absolute risk of MACE, MALE, or both in order to identify those who will derive the greatest absolute benefits relative to the risk of bleeding.
Patients with polyvascular disease including symptomatic CAD and especially prior MI have been shown to be at the highest risk of MACE and derive robust benefits for MACE with more potent strategies. The COMPASS trial enriched for polyvascular disease and overall 90% had coronary disease with 69% of those with PAD having prior MI or stroke. Therefore, symptomatic coronary disease would be reasonable to identify a high-risk group for MACE likely to derive a robust benefit of therapy. In addition, patients with diabetes have a higher risk of MACE and are likely to derive greater benefit. This was shown with vorapaxar in the TRA2P-TIMI 50 trial.
In identifying patients at risk for MALE, the most important predictor in three studies is a prior peripheral revascularization procedure. There is a 4-fold risk of MALE in patients with a history of prior intervention even if several years prior. In the COMPASS trial, the highest risk of MALE was similarly in those with prior intervention, with only 5 events in the 1422 patients with asymptomatic low ABI and 86 events in the 2264 with prior revascularization or amputation.

Therefore, assessing a patient’s risk of MACE and MALE will help to identify those most likely to benefit from more potent antithrombotic strategies. A flow chart for suggested decision-making is shown below.

The agents selected may depend on the profile of the patient. Given the broad benefits shown in the COMPASS trial, the addition of rivaroxaban to aspirin monotherapy may be attractive to those patients who can receive it. For patients with prior MI, particularly a recent MI or who need P2Y12 inhibition due to recent intervention, ticagrelor 60 mg twice daily is another option. The risks and benefits of these therapies need to be carefully considered with the patient in shared decision-making.
